

There is currently no FDA-approved or FDA-authorized COVID-19 vaccine for children younger than age 6 months.
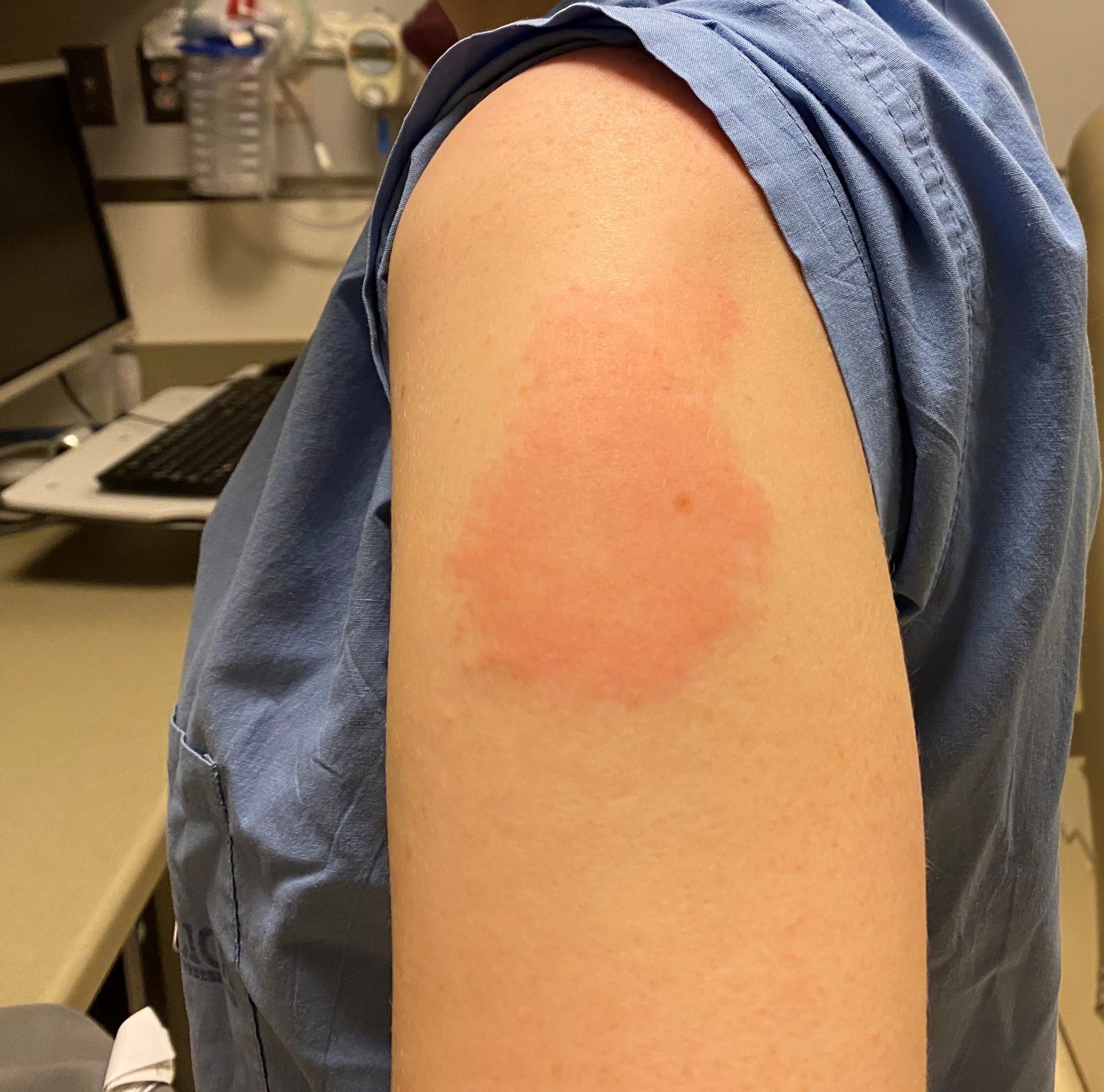
Covid vaccine after effects duration full#
COVID-19 Vaccine Product Information can be consulted for a full list of ingredients and information on the conditions of use, storage and handling, preparation, and administration procedures.ĬOVID-19 vaccination is recommended for everyone ages 6 months and older in the United States for the prevention of COVID-19.
Novavax COVID-19 Vaccine is currently formulated as a monovalent vaccine based on the Original strain of SARS-CoV-2.ĬOVID-19 vaccine-specific package inserts and FDA fact sheets and U.S.The bivalent formulation (Original and Omicron BA.4/BA.5) should no longer be used. Moderna and Pfizer-BioNTech COVID-19 vaccines: The 2023–2024 formulation has been updated to a monovalent vaccine based on the Omicron XBB.1.5 sublineage of SARS-CoV-2.Novavax COVID-19 Vaccine, Adjuvanted (hereafter referred to as Novavax COVID-19 Vaccine) is authorized for people ages 12 years and older for primary vaccination and, in certain limited situations, as a booster dose in people ages 18 years and older.These vaccines are hereafter referred to as updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 Vaccine. Pfizer-BioNTech COVID-19 Vaccine (2023–2024 Formula) is authorized for children ages 6 months–11 years COMIRNATY is the licensed Pfizer-BioNTech product for people ages 12 years and older.These vaccines are hereafter referred to as updated (2023–2024 Formula) Moderna COVID-19 Vaccine. Moderna COVID-19 Vaccine (2023–2024 Formula) is authorized for children ages 6 months–11 years SPIKEVAX is the licensed Moderna product for people ages 12 years and older.Two types of COVID-19 vaccines are available for use in the United States: Reorganization and consolidation of sections on contraindications and precautions, including allergic reactions to COVID-19 vaccines.Updated guidance for COVID-19 vaccination and Multisystem Inflammatory Syndrome (MIS) in children (MIS-C) and in adults (MIS-A).Updated guidance for COVID-19 vaccination and myocarditis or pericarditis.Bivalent mRNA COVID-19 vaccines are no longer recommended in the United States.May receive 1 or more additional updated (2023–2024 Formula) mRNA COVID-19 vaccine doses.Received previous mRNA doses: need 1 or 2 doses of updated (2023–2024 Formula) Moderna or updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 vaccine, depending on the number of prior doses.

Covid vaccine after effects duration series#
Initial vaccination: should receive a 3-dose series of updated (2023–2024 Formula) Moderna or updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 vaccine.People who are moderately or severely immunocompromised.Received previous mRNA doses: need 1 or 2 doses of updated (2023–2024 Formula) Moderna or updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 vaccine, depending on the number of prior doses.Initial vaccination: should receive either 2 doses of updated (2023–2024 Formula) Moderna or 3 doses of updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 vaccine.Everyone ages 5 years and older is recommended to receive 1 dose of updated (2023–2024 Formula) mRNA COVID-19 vaccine.Recommendations for use of the 2023–2024 formulations of Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine:.Summary of recent changes (last updated September 15, 2023):


 0 kommentar(er)
0 kommentar(er)
